Manitoba
Human Tissue Gift Act
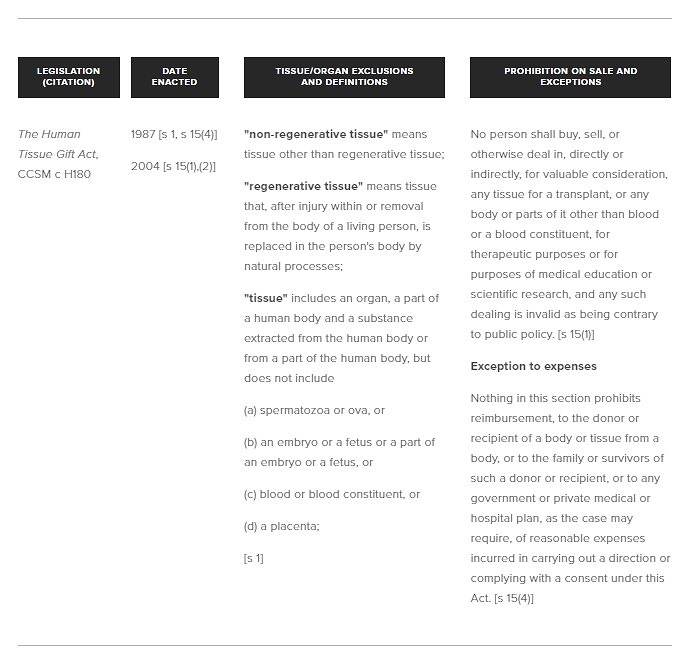
In 1987, Manitoba was the first to exclude embryos, fetus, sperm, ova and placenta from the application of their Human Tissue Gift Act. The amendments were a result of recommendations made by the Law Reform Commission of Manitoba since Manitoba’s organ and tissue donation legislation had not changed since adopting the 1965 Uniform Human Tissue Act. The Commission recommended that Manitoba not adopt the 1971 Uniform Human Tissue Act, finding that the 1971 legislation failed to deal with “regenerative” tissue donation.
Use of the term “regenerative” tissue allows for the differentiation between the temporary loss of tissue like blood, bone marrow, skin or sperm, which regenerates itself, versus non-regenerative tissue, like kidneys, which are irreplaceable and are permanently lost after donation. The rationale for treating the two types of tissue differently is because of the difference in the supposed risk and permanent loss in donating. The Uniform Law Conference of Canada presumably chose not to include regenerative tissue in their 1971 legislation because the risk of harm in donating regenerative tissue is less. However, the Law Reform Commission of Manitoba felt that regenerative tissues, particularly bone marrow and skin, should be included in the legislation due to the risk of harm as a result of the painful donation process of these tissues.[i]
The decision to exclude spermatozoa and ova, however, is a separate discussion from regenerative versus non-regenerative tissues. In the case of gametes(sperm and ova), embryos and fetal tissue, the consensus appears to be that greater value should be placed on these reproductive tissues as compared to human tissue generally. Due to the greater value placed on these tissues, the legal and ethical issues are more complex and therefore the decision was made to not include them in the Human Tissue Gift Act. The Manitoba Law Reform Commission also maintained the exclusion of blood and blood constituents from the legislation since it was felt that new legislation was not needed given the already routine nature of blood donation.[ii]
The Commission also recommended maintaining the prohibition on the sale of tissue. Reasoning provided for the prohibition include that allowing the sale of human tissue would only encourage blackmail, coercion or duress; increase the possibility of donors lying or concealing health defects (thus increasing the danger to recipients); as well as wrongly encourage donations from the poor. However, blood and blood constituents were excluded from the prohibition.
The Government of Manitoba followed the Commission’s recommendations, but also added placenta to the tissue exemption list because they
were advised by representatives from the medical profession that in fact there is a provision later on that has to do with the question of the use of human tissue, and the placenta has a number of uses. One of the primary medical uses for the placenta is the extraction of gamma globulin, so we felt it was necessary to make sure there was a specific reference to placenta.[iii]
Notably, Manitoba has attempted to define what constitutes “sale” of tissue by creating an exception for “reasonable expenses incurred” in donating tissue or organs. There is some ambiguity, however, for what may be considered reasonable. For example, whether that includes remuneration for the time taken to donate tissue, or salary lost due to providing the service, is not clear. Additionally, blood and blood constituents remain clearly exempt from the prohibition, as Manitoba has historically provided small honorariums for donations of rare blood types.[iv] As with other provinces, the wording of the prohibition on sale applies to “any body or parts”, suggesting that all tissues, even those excluded, may still fall under the prohibition, however, this is arguable.
For Profit Plasma Clinic
Due to the exclusion of blood from the definition of tissue and explicitly from the prohibition on sale in the Human Tissue Donation Act, as of 2024 there is a pay-for-plasma clinic open in Winnipeg.
References
[i] Law Reform Commission of Manitoba, Report on The Human Tissue Act, Report 66 (1986). [online: www.manitobalawreform.ca/pubs/pdf/archives/66-full_report.pdf ]
[ii] See above.
[iii] Legislative Assembly of Manitoba, Standing Committee on Statutory Regulations and Orders, “Bill 40, The Human Tissue Act”, 2nd Reading, Statutory Regulations and Orders, 33:2, vol 3 (14 July 1987) at 88 (Hon R Penner).
[iv] Commission of Inquiry on the Blood System in Canada: Final Report, vol 1 (Ottawa: Public Works and Government Services Canada, 1997). [online: publications.gc.ca/site/eng/9.698032/publication.html ]